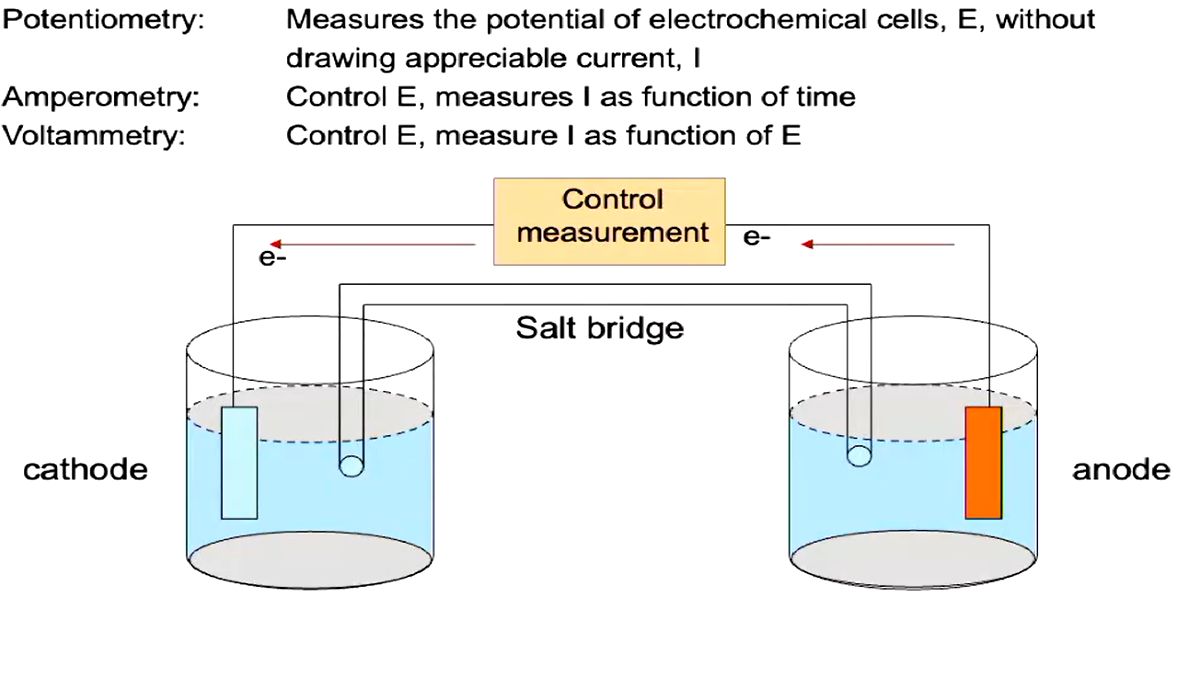
 reduction occurs at the anode (electrons are deposited onto the anode)
reduction occurs at the anode (electrons are deposited onto the anode)
oxidation occurs at the cathode (electrons are lost from the cathode)
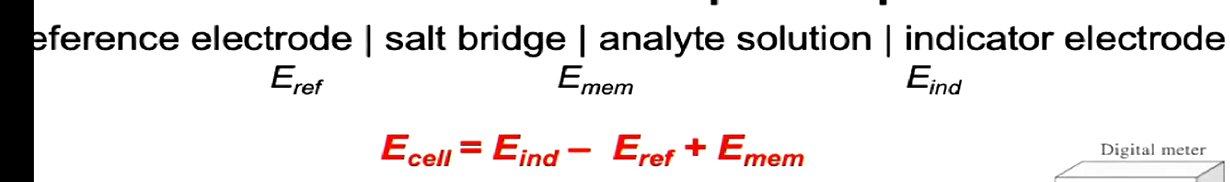
Cell Notation
Anode (s) | solution (aq) || solution (aq) | Cathode (s)
anode half cell || cathode half Cell
-> direction of electron flow ->
E(cell) = E(cathode) - E(anode)